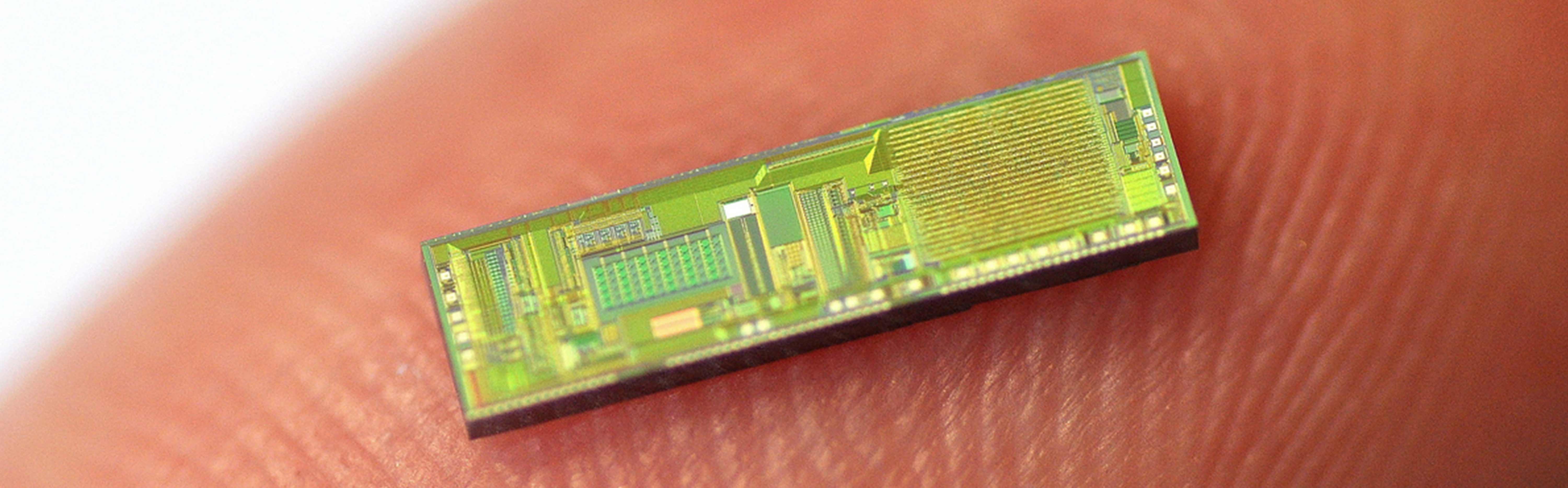
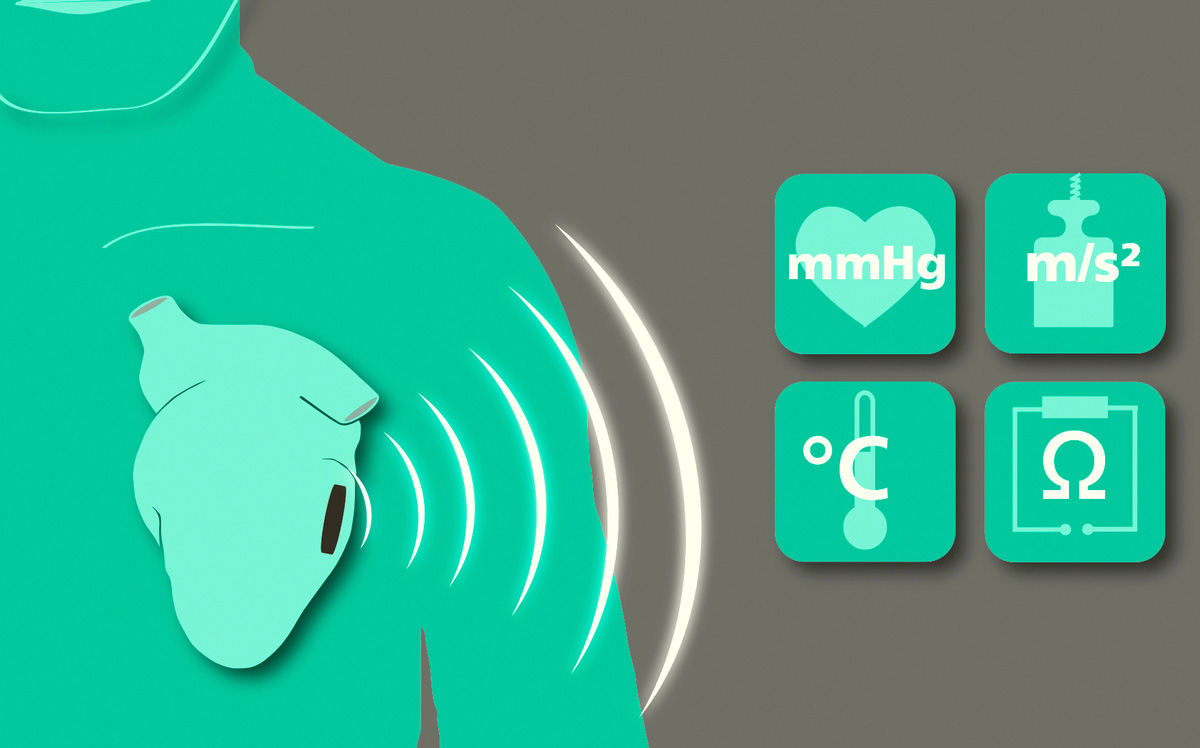
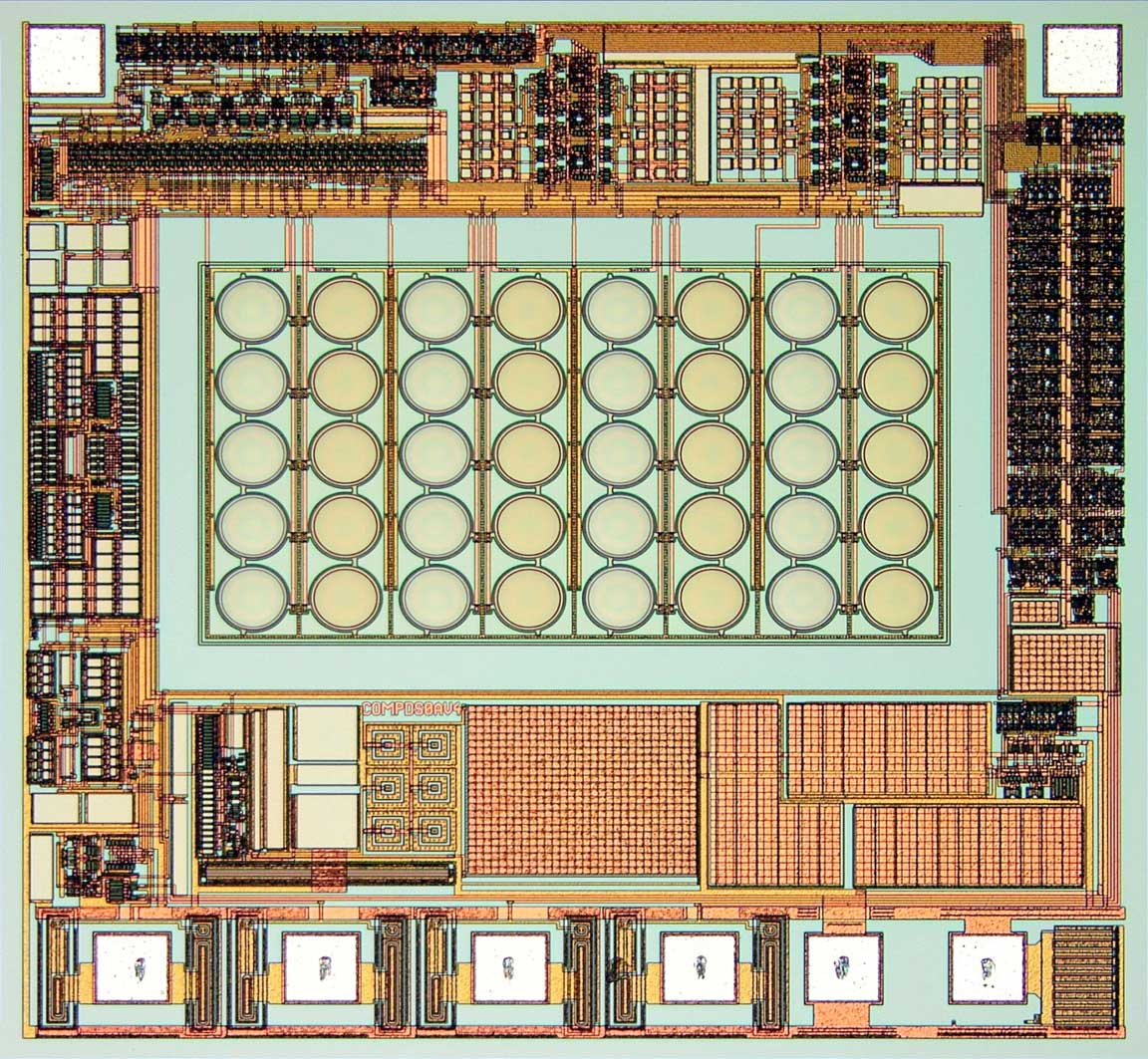
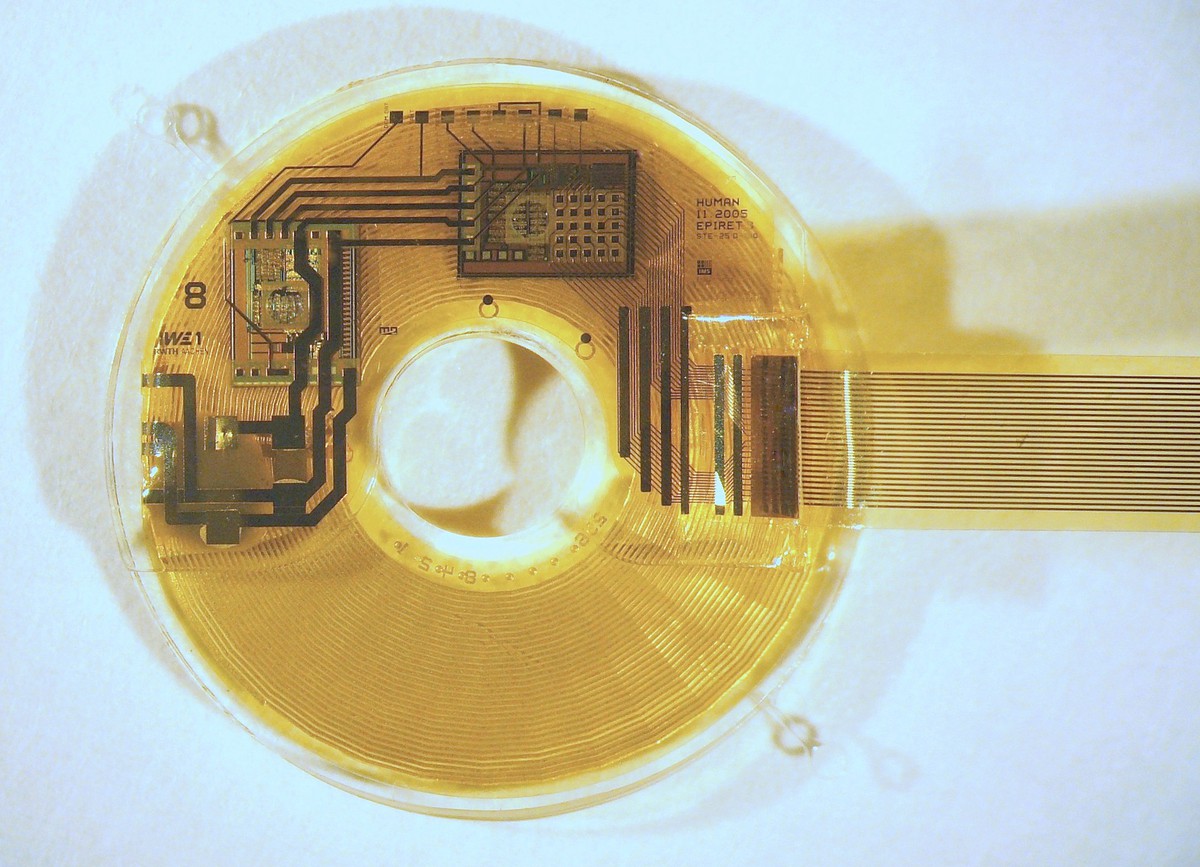
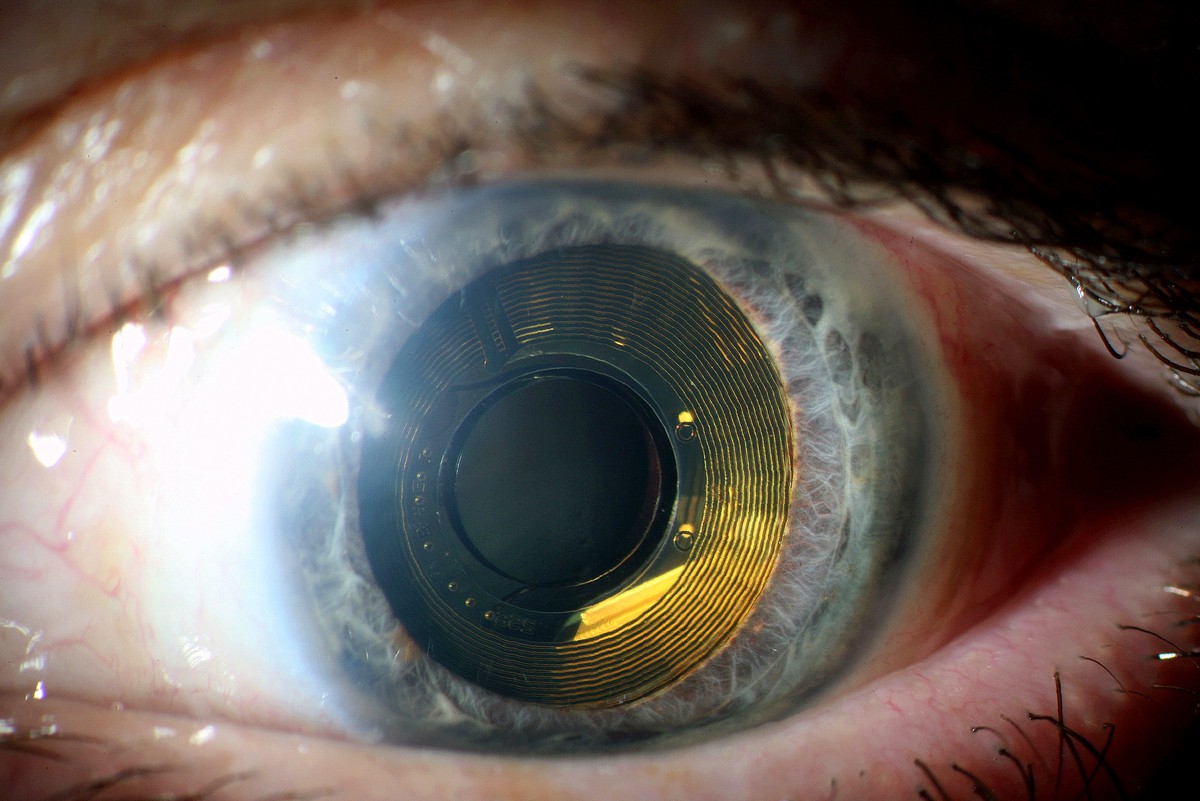
Fraunhofer IMS is developing the next generation of intelligent closed-loop implants. They record the wearer's vital signs and, on this basis, can initiate therapeutic measures in a closed loop to improve the patient's health.
The trend towards continuous miniaturization of medical Implants is intended to open up new, previously unfeasible applications. In addition, an optimized overall performance is to be achieved for existing applications through increased functionality. These challenges are the elementary specifications for the development of highly complex implantable systems consisting of sensors, actuators, efficient electronics, sensor-related evaluation by AI algorithms, secure communication, long-term energy supply and biostable encapsulation.